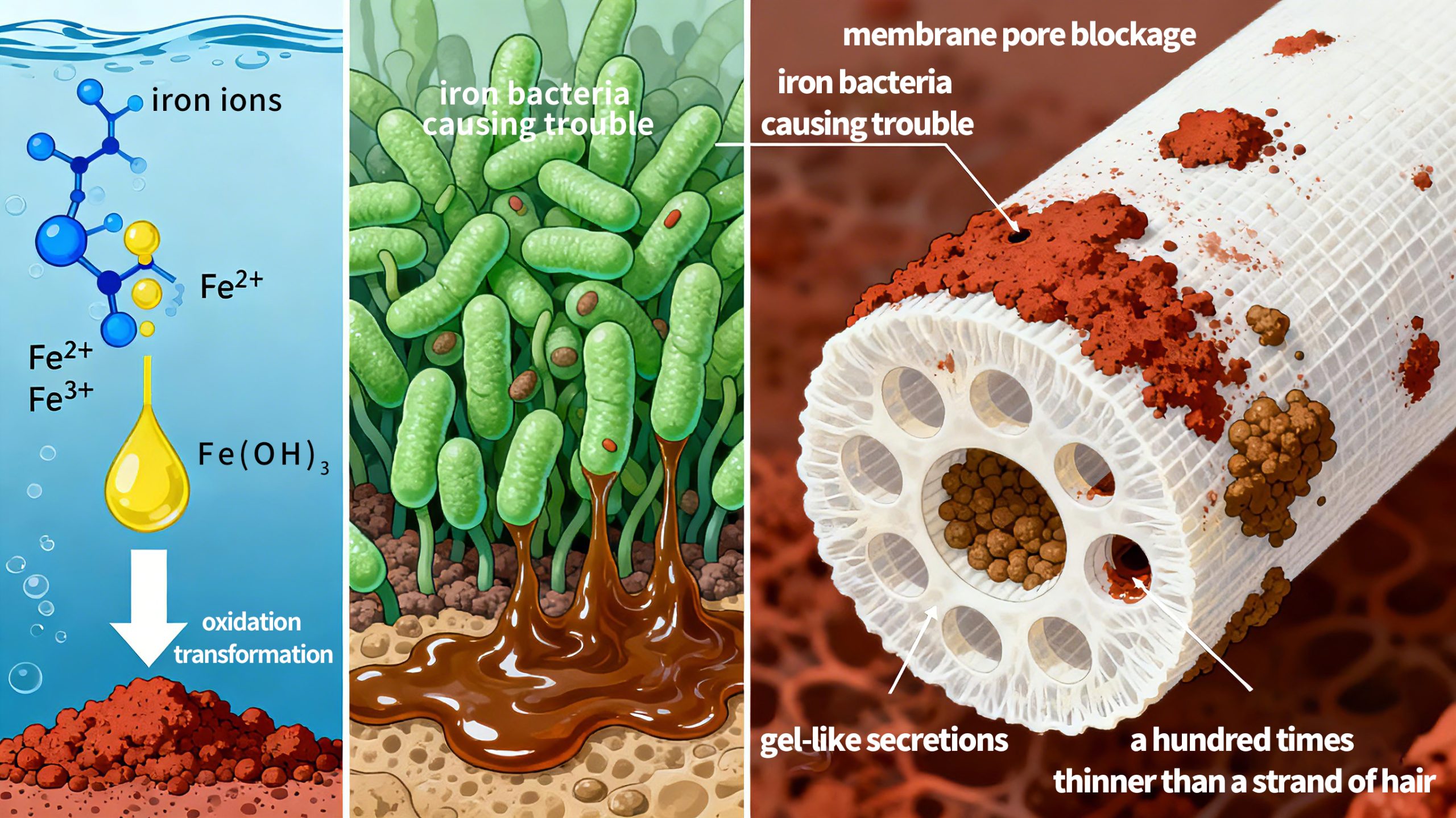
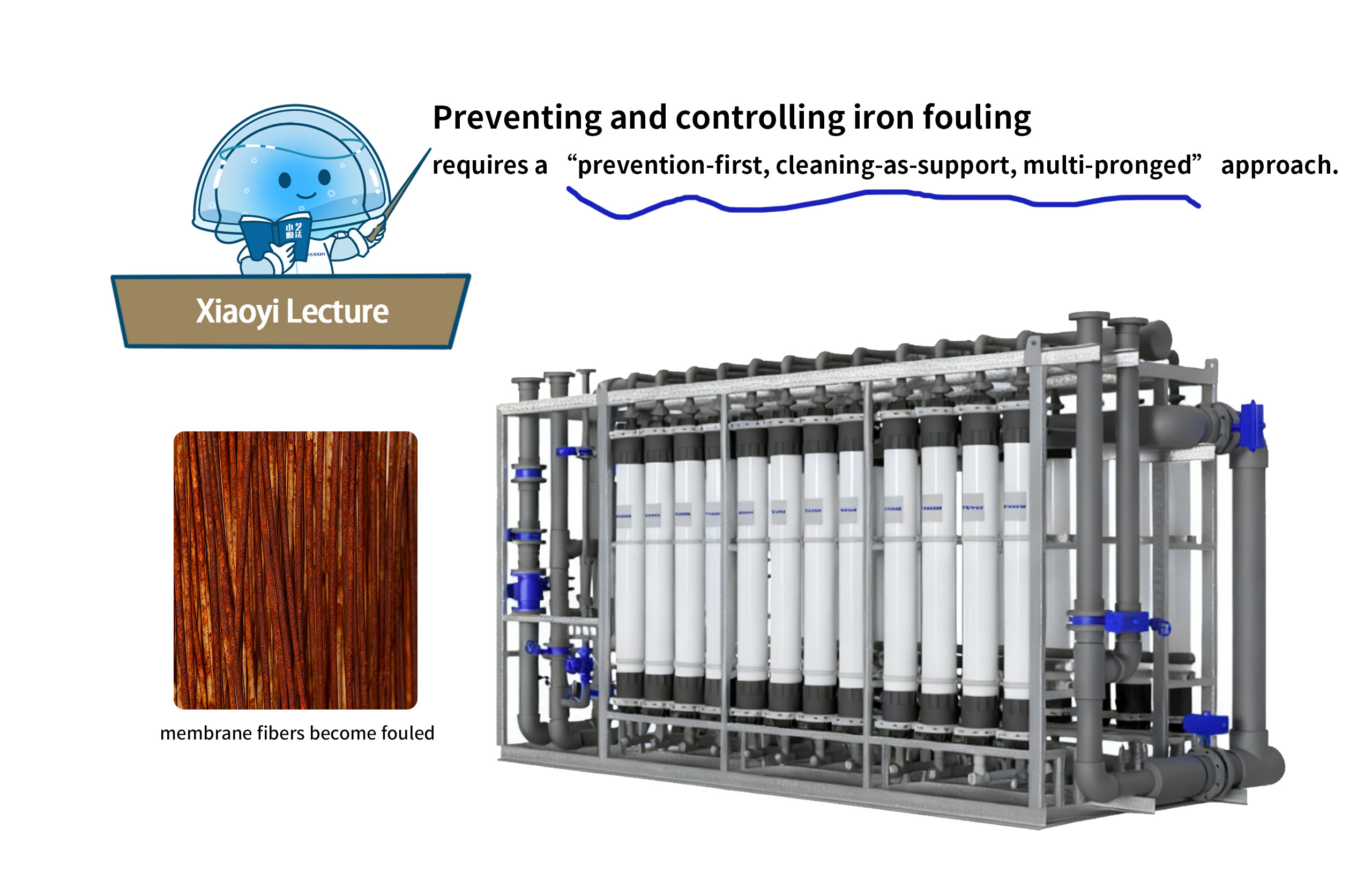
Image a pressurized ultrafiltration membrane wrapped tightly in a stubborn reddish-brown “rust” layer, with water production decreasing and water quality deteriorating. This is the “iron fouling” crisis faced by pressurized ultrafiltration membrane systems. Such fouling not only reduces water production efficiency but also shortens membrane lifespan and increases operational costs.
Today, let’s dive into the causes of iron fouling and explore how Shuiyi Membrane Technology scientifically prevents and controls iron fouling in ultrafiltration membranes.

Part 01 Iron Fouling: The “Rust” Crisis Inside the Membrane Pores
The essence of iron fouling is that dissolved iron ions in water (primarily ferrous iron Fe²⁺) transform under specific conditions into insoluble “rust” (ferric iron compounds such as Fe(OH)₃), clogging the pores of the ultrafiltration membrane.
Part 02 Who Is “Adding Fuel to the Fire”? Key Contributing Factors of Iron Fouling
01 Iron Content Level
The higher the total iron concentration in water (especially > 0.3 mg/L), the significantly greater the fouling risk, making it a decisive factor affecting fouling severity.
02 Oxidation “Catalysts”
Dissolved oxygen (DO), oxidants (such as chlorine), and the presence of iron bacteria, etc.
03 System Operating Conditions
Operating pressure and flow velocity, backwash/air scouring effectiveness, etc.
04 Water Quality “Accomplices”
Organic matter (TOC), silica and hardness, and pH value (higher pH is more conducive to the formation and stability of Fe(OH)₃ precipitates).
Part 03 Building Defenses: Scientific Strategies for Preventing and Controlling Iron Fouling
01 Source Interception: Strengthened Pre-treatment (the Fundamental Solution)
- Aeration oxidation + filtration: For groundwater containing iron, use aeration towers or cascading aeration to fully expose water to air, oxidizing Fe²⁺to Fe³⁺, followed by removal of precipitates using sand filters or multimedia filters. This is the most classic and effective method.
- Chemical oxidation + sedimentation: Add oxidants such as sodium hypochlorite, potassium permanganate, or ozone to rapidly oxidize Fe²⁺, then remove iron sludge through sedimentation tanks or filters.
- Ion exchange/ softening: Under specific conditions (e.g., low iron content coexisting with hardness), softening resins may be used to remove some iron ions (especially bound iron).
02 System Operation Optimization (Reducing Deposition Opportunities)
- Strict control of oxidant dosing: Strive to complete iron oxidation and removal during pretreatment to avoid oxidants entering the membrane system. If chlorine must be dosed before the membrane, strictly control the dosage and use activated carbon filters or reducing agents (e.g., sodium bisulfite) to ensure dichlorination.
- Optimize operating parameters: While ensuring flux, adopt appropriate operating pressure and crossflow velocity to reduce the tendency of pollutants to deposit on the membrane surface.
- Strengthen physical cleaning: Ensure sufficient backwash frequency, duration and intensity. Make effective use of air scouring, as bubble scrubbing effectively removes soft fouling such as iron sludge and biofilm. This is key to daily maintenance.
03 Final Measure: Chemical Cleaning (Removing Existing Fouling)
- Acid cleaning (targets inorganic iron scale): Use citric acid, oxalic acid, or dilute hydrochloric acid (common concentration 0.1%-2%). Acids effectively dissolve inorganic precipitates such as ferric hydroxide and iron oxide. Pay attention to the corrosiveness of acids on metal fittings, and strictly control concentration, pH and contact time.
- Alkaline cleaning (targets organics/biofilm): Use sodium hydroxide (commonly concentration 0.01% -0.1%) or combine with surfactants/chelating agents (such as EDTA). Alkali can saponify organic matter and disrupt biofilm structures, making it particularly effective against fouling caused by iron bacteria.
- Targeted Biocides: If severe iron bacteria fouling is confirmed, add non-oxidizing biocides (such as isothiazolinones, DBNPA) to the alkaline cleaning solution or use them separately during maintenance cleaning to kill and remove biofilms.
- Cleaning Strategy: Follow the principle of “low concentration, short duration, high frequency”, which is safer and more effective than “high concentration, long duration, low frequency”. The cleaning procedure (order of acid/alkaline cleaning, soaking time, temperature) should be scientifically designed based on the specific composition and severityof the fouling.
Part 04 Multiple Measures Working Together: Keeping Ultrafiltration Systems Free from Iron Fouling
Iron fouling is a major threat to the stable operation of pressurized ultrafiltration membrane systems. Understanding oxidation, deposition and biofilm formation mechanisms and monitoring iron levels, oxidation conditions, and operating parameters forms the foundation of effective prevention and control.
Shuiyi Membrane Technology adopts a comprehensive strategy, including strengthening pretreatment for iron removal (aeration filtration/manganese sand filtration), optimizing operations to avoid pre-membrane oxidation, enhancing physical cleaning (backwashing + air scouring) and scientifically conducting chemical cleaning (acid cleaning + alkaline cleaning + biocides). This can maximize control over iron fouling, ensuring efficient, stable and long-lasting operation of ultrafiltration membrane systems, allowing every drop of clean water flow unimpeded.
Part 05 Typical Ultrafiltration Membrane Projects by Shuiyi Membrane Technology
01

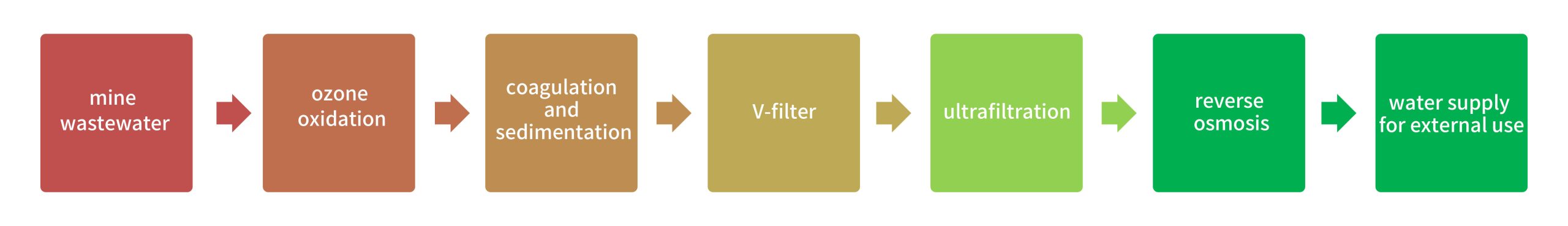
Project Name: Wastewater reuse project at a coal mine in Ningxia
Capacity: 30,000 T/D
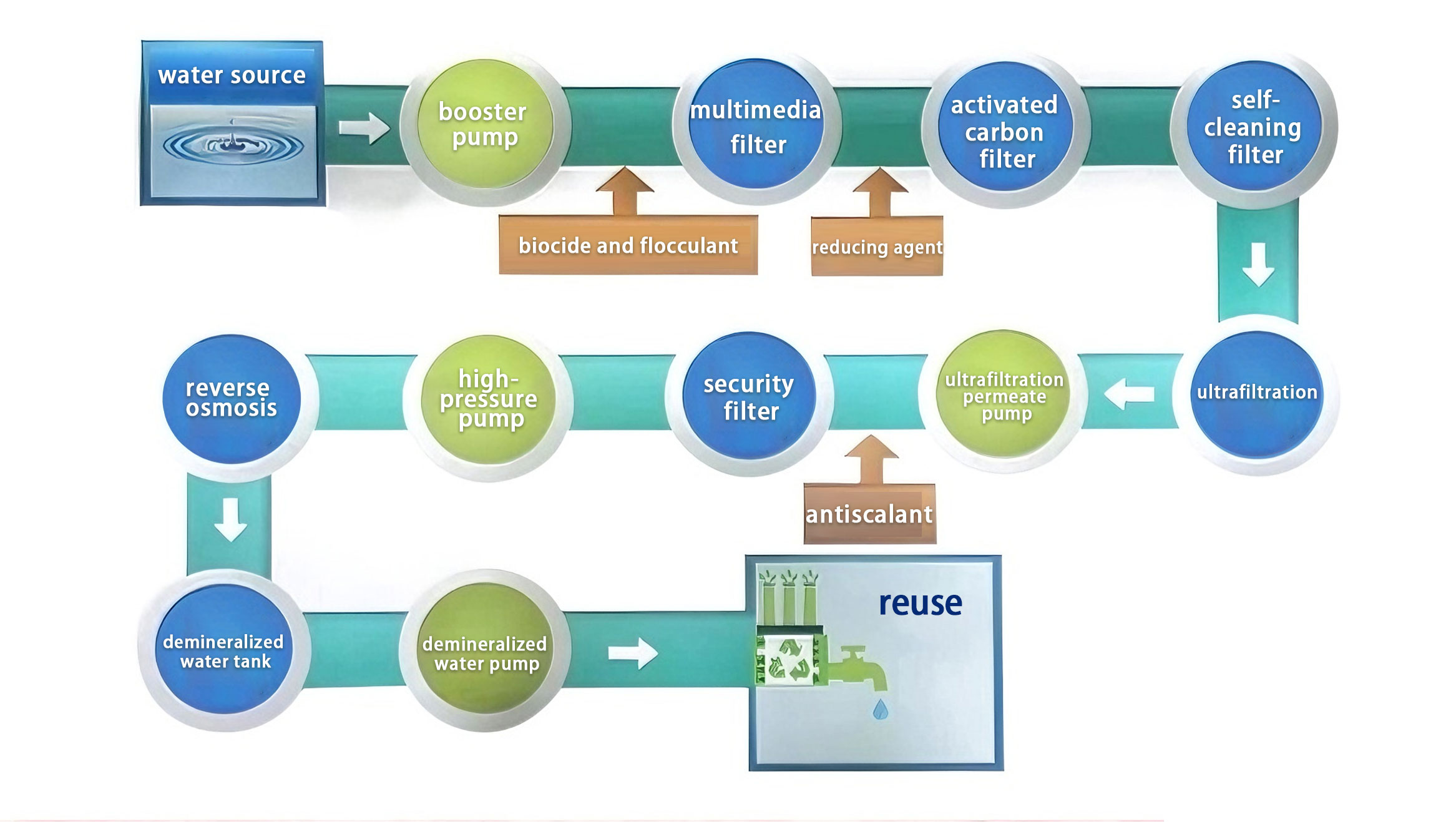
Process: Ozone oxidation + coagulation and sedimentation + V-filter + ultrafiltration + reverse osmosis
The produced water meets GB50050-2017 Design Code for Industrial Circulating Cooling Water Treatment and is supplied to nearby power plants.
02

Project Name: Demineralized project for a steel group in Hebei province
Capacity: 9600T/D
Process: Multimedia filtration → activated carbon filtration → ultrafiltration → reverse osmosis